Cranial Remodeling
Number: 0379
Table Of Contents
PolicyApplicable CPT / HCPCS / ICD-10 Codes
Background
References
Policy
Scope of Policy
This Clinical Policy Bulletin addresses cranial remodeling.
-
Medical Necessity
-
Aetna considers cranial remodeling bands (or helmets) as medically necessary orthoses for treatment of moderate-to-severe positional head deformities associated with premature birth, restrictive intra-uterine positioning, cervical abnormalities, birth trauma, torticollis (shortening of the sternocleidomastoid muscle) and sleeping positions in children when banding is initiated at 3 to 12 months of age and the following conditions are met:
- A 2-month trial of conservative therapy consisting of re-positioning the child's head such that the child lies opposite to the preferred position, has failed to improve the deformity and is judged to be unlikely to do so, and
- One of the following must be met:
-
Anthropometric data (measurements used to evaluate abnormal head shape by measuring the distance in mm from one pre-designated point on the face or skull to another, comparing the right and left sides) verifies that a moderate-to-severe plagiocephaly is documented by a physician experienced in such measurement. Note: These measurements are generally obtained by the orthotist fitting the band or helmet. Medical notes documenting at least one the following is needed: cephalic index (CI), Children’s Healthcare of Atlanta (CHOA) level, cranial vault asymmetry (CVA), cranial vault asymmetry index (CVAI), orbitotragial depth asymmetry (OTDA), skull base asymmetry (SBA), and transcranial diameter difference (TDD).
Diagram:
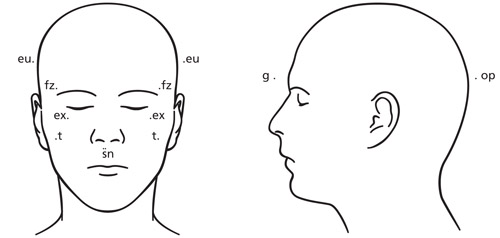
A difference of asymmetry greater than 6 mm between anthropometric measurements (see diagram above) in any of the anthropometric data in the first column of the following table warrants coverage of a trial of orthotic banding to correct the craniofacial deformity:
Table 1: Anthropometric Data Measurements and Measures Anthropometric Data Measurement Measures Cranial base
(sn-t on same side)from right and left subnasal point (sn) to tragus (t) measures maxillary depth or right and left morphological face height Cranial vault
(fz R-euL, fz L-euR)from frontozygomaticus point (fz) on one side of face to euryon (eu) measures cranial vault asymmetry Orbitotragial depth
(ex-t, R, L)from exocanthion point (ex) to tragus (t) measures orbito-tragion depth (exocanthion) -
For brachycephaly evaluation, a cephalic index of 2 standard deviations (SDs) below mean (head narrow for its length) or 2 SDs above mean (head wide for its length) warrants coverage of a trial of orthotic banding to correct the craniofacial deformity in a child after 4 months of age and before 12 months of age. (Note: These measurements are generally obtained by the orthotist fitting the band or helmet).
Table 2: Brachycephaly Evaluation - Measurements and Measures Brachycephaly Evaluation Data Measurement Measures Head width
(eu - eu)from euryon (eu) on one side of head to euryon (eu) on the other side measures greatest transverse diameter or maximal head width Head length
(g-op)from glabella point (g) to opisthocranion (op) measures maximal head depth or length Cephalic index equal to head width eu minus eu multiplied by 100 divided by head length g minus op
Table 3: Cephalic Index for Male and Female and their age Sex Age -2 SD -1 SD Mean +1 SD +2 SD Male 16 days to 6 months 63.7
68.7
73.7
78.7
83.7
Male 6 to 12 months
64.8
71.4
78.0
84.6
91.2
Female 16 days to 6 months 63.9
68.6
73.3
78.0
82.7
Female 6 to 12 months 69.5
74.0
78.5
83.0
87.5
- Infants who develop significant plagiocephaly secondary to a constant head position required for long-term hyperalimentation who do not respond to simple changing of the catheter location allowing the head to be re-positioned.
- Members with excess frontal bossing secondary to sagittal synostosis
- Premature infants with dolichocephalic head shape who have developed a mis-shapen head secondary to sustained head position.
A second cranial remodeling band or helmet is considered medically necessary for children who met the aforementioned criteria at the initiation of therapy if the asymmetry has not resolved or significantly improved after 2 to 4 months such that the severity of head deformity indicates another orthosis and the orthosis becomes ill-fitting after attempts to adjust and leaves little or no room for new growth. A second orthosis may be medically necessary to prevent regression of head shape in very young infants (8 months or younger) who met the aforementioned criteria at the initiation of therapy, who have outgrown the initial orthosis, and have not developed midline head control, rolling, or sitting. Note: Remodeling bands (or helmets) are contraindicated and considered not medically necessary after 2 years of age.
-
- Aetna considers the use of a cranial remodeling band (or helmet) cosmetic for persons not meeting the aforementioned criteria.
- Aetna considers use of a cranial remodeling band (or helmet) medically necessary for infants with synostotic plagiocephaly to correct continued asymmetry following surgery (i.e., a trial of conservative therapy is not needed when the cranial remodeling band is used following surgery for synostotic plagiocephaly).
- Aetna considers distraction osteogenesis medically necessary for syndromic craniosynostosis – Apert, Crouzon, and Pfeiffer syndromes.
Note: Aetna considers cranial remodeling helmets and bands contraindicated and not medically necessary in unshunted or uncontrolled hydrocephalus.
-
-
Experimental and Investigational
The following procedures are considered experimental and investigational because the effectiveness of these approaches has not been established:
- Intra-operative indocyanine green angiography to evaluate scalp perfusion during cranial vault remodeling in infants
- Pre-operative molding helmet therapy for the treatment of sagittal craniosynostosis
- Use of a cranial remodeling band or helmet for calcified cephalohematoma
- Use of a cranial remodeling band or helmet without surgery to correct asymmetry in infants with synostotic plagiocephaly; craniosynostosis that is not surgically corrected is a contraindication to use of cranial remodeling bands or helmets.
- Use of polycaprolactone mesh in pediatric cranial vault remodeling surgery
- Use of sleep positioning wrap for the treatment of infants with positional head shape deformities.
-
Related Policies
| Code | Code Description |
|---|---|
CPT codes covered if selection criteria are met: |
|
| 20690 | Application of a uniplane (pins or wires in 1 plane), unilateral, external fixation system |
| 20692 | Application of a multiplane (pins or wires in more than 1 plane), unilateral, external fixation system (eg, Ilizarov, Monticelli type) |
| 20693 | Adjustment or revision of external fixation system requiring anesthesia (eg, new pin[s] or wire[s] and/or new ring[s] or bar[s]) |
| 20694 | Removal, under anesthesia, of external fixation system |
| 20696 | Application of multiplane (pins or wires in more than 1 plane), unilateral, external fixation with stereotactic computer-assisted adjustment (eg, spatial frame), including imaging; initial and subsequent alignment(s), assessment(s), and computation(s) of adjustment schedule(s) |
| 20697 | Application of multiplane (pins or wires in more than 1 plane), unilateral, external fixation with stereotactic computer-assisted adjustment (eg, spatial frame), including imaging; exchange (ie, removal and replacement) of strut, each |
CPT codes not covered for indications listed in the CPB: |
|
| 92240 | Indocyanine-green angiography (includes imaging) with interpretation and report |
| 92242 | Fluorescein angiography and indocyanine-green angiography (includes multiframe imaging) performed at the same patient encounter with interpretation and report, unilateral or bilateral |
Other CPT codes related to the CPB: |
|
| 61550 | Craniectomy for craniosynostosis; single cranial suture |
| 61552 | multiple cranial sutures |
| 61556 | frontal or parietal bone flap |
| 61557 | bifrontal bone flap |
| 61558 | Extensive craniectomy for multiple cranial suture craniosynostosis (eg, cloverleaf skull); not requiring bone grafts |
| 61559 | recontouring with multiple osteotomies and bone autografts (eg, barrel-stave procedure) (includes obtaining grafts) |
| 97763 | Orthotic(s)/prosthetic(s) management and/or training, upper extremity(ies), lower extremity(ies), and/or trunk, subsequent orthotic(s)/prosthetic(s) encounter, each 15 minutes |
HCPCS codes covered if selection criteria are met: |
|
| D5924 | Cranial prosthesis |
| L0112 | Cranial cervical orthosis, congenital torticollis type, with or without soft interface material, adjustable range of motion joint, custom fabricated |
| L0113 | Cranial cervical orthotic, torticollis type, with or without joint, with or without soft interface material, prefabricated, includes fitting and adjustment |
| S1040 | Cranial remolding orthosis, pediatric, rigid, with soft interface material, custom fabricated, includes fitting and adjustment(s) |
HCPCS codes not covered for indications listed in the CPB: |
|
| C1781 | Mesh (implantable) [polycaprolactone mesh] |
| C9733 | Non-ophthalmic fluorescent vascular angiography |
ICD-10 codes covered if selection criteria are met: |
|
| Q67.2 | Dolichocephaly |
| Q67.3 | Plagiocephaly |
| Q67.4 | Other congenital deformities of skull, face and jaw (see criteria) |
| Q75.0 | Craniosynostosis [not surgically corrected is a contraindication to use of cranial remodeling bands or helmets] |
| Q75.1 | Craniofacial dysostosis [Crouzon's disease] |
| Q75.8 - Q75.9 | Other specified congenital malformations of skull and face bones (see criteria) |
ICD-10 codes not covered for indications listed in the CPB (not all inclusive): |
|
| G91.0 | Communicating hydrocephalus [unshunted or uncontrolled] |
| G91.1 | Obstructive hydrocephalus [unshunted or uncontrolled] |
| G91.2 | (Idiopathic) normal pressure hydrocephalus [unshunted or uncontrolled] |
| P12.0 | Cephalohematoma due to birth injury [calcified] |
| Q03.0 - Q03.9 | Congenital hydrocephalus [unshunted or uncontrolled] |
| Q05.0 - Q05.4 | Spina bifida with hydrocephalus [unshunted or uncontrolled] |
Background
Plagiocephaly (an asymmetrical head shape) is most often the result of an infant spending extended periods of time on their back, typically during sleep. Plagiocephaly can also occur as a feature of other disorders (e.g., craniofacial disorders, torticollis, cervical anomalies) and is categorized as either positional or synostotic (premature union of cranial sutures). Although 1 in 300 infants exhibit variable degrees of plagiocephaly, true sutural synostosis, which interferes with cranium development and may cause increased intra-cranial pressure, occurs in only 0.4 to 1 per 1,000 live births.
Positional plagiocephaly is treated conservatively and many cases do not require any treatment as the condition may resolve spontaneously when the infant begins to sit up. When the deformity is moderate or severe and a trial of re-positioning the infant has failed, a pediatric neurologist, neurosurgeon or other appropriate specialist in craniofacial deformities may prescribe a cranial remodeling band to remodel the misshapen head.
Cranial orthotics are designed to improve plagiocephaly without synostosis or deformational plagiocephaly, which is a condition found in infants whose heads show an asymmetrical flattening caused by uneven external pressures on the skull. There are two types of cranial orthotics: cranial bands and soft-shell helmets.
The headband or helmet is custom made and custom fitted to the infant’s head and is designed to actively guide the growth of the skull to a more normal shape. Orthotic management of plagiocephaly without synostosis is usually initiated between three and 18 months of age and continues for an average of four to six months. Both helmets and cranial bands are recommended to be worn 23 hours per day with an hour off for exercises and skin care.
Examples of brands of cranial remodeling bands and helmets include the DOC BAND®, Gillette Children's Craniocap, and the STARband™ Cranial Headband. Average treatment time with the cranial remodeling band or helmet is 4.5 months.
A systematic evidence review of cranial orthosis treatment for infant deformational plagiocephaly prepared for the UK National Health Services (NHS QIS, 2007) found no randomized controlled trials assessing the effectiveness of cranial orthoses for the treatment of deformational plagiocephaly were identified. The assessment stated that no evidence-based conclusions can be reached on the effectiveness of cranial orthoses due to the limited methodological quality of the available trials. "Further research in the form of a randomised controlled trial is needed to determine the true effectiveness of cranial orthoses."
While infants with positional plagiocephaly may be treated with head positioning and/or helmeting, the standard treatment for synostotic plagiocephaly (asymmetrical head caused by premature closure of the cranial sutures) is surgery. There is some evidence suggesting that a cranial remodeling band (or helmet) may improve outcomes following surgery to treat synostotic plagiocephaly. Seymour-Dempsey et al (2002) retrospectively reviewed the results of surgery alone (n = 6) versus surgery and post-operative banding (n = 15) in treating children diagnosed with sagittal synostosis. The investigators reported that correction toward a normal cephalic index was seen in the banded group throughout the course of treatment, while this trend was not present in the non-banded group.
Cranial remodeling bands and helmets are contraindicated in infants older than 24 months. The skulls of these children have finished growing and no longer have the pliability and plasticity necessary to create a change in shape.
In a randomized controlled trial, Hutchison et al (2010) examined the effectiveness of the Safe T Sleep positioning wrap in infants with positional head shape deformities. A total of 126 infants presenting to a plagiocephaly clinic were randomized to either positioning strategies or to positioning plus the use of a Safe T Sleep™ positioning wrap. Head shape was measured using a digital photographic technique, and neck function was assessed. They were followed-up at home 3, 6 and 12 months later. There was no difference in head shape outcomes for the 2 treatment groups after 12 months of follow-up, with 42 % of infants having head shapes in the normal range by that time; 80 % of children showed good improvement. Those that had poor improvement were more likely to have both plagiocephaly and brachycephaly and to have presented later to clinic. The authors concluded that most infants improved over the 12-month study period, although the use of a sleep positioning wrap did not increase the rate of improvement.
Larsen (2004) stated that a second orthosis is rarely required but could be used in very severe head deformations, unusual circumstances (illness-negated use or if the child has serious health and/or positioning issues), or unusually high expectations of the family. The author noted that criteria for determining a second orthosis include the following:
- Despite every effort, the orthosis becomes ill-fitting or leaves little or no room for new growth;
- If age and severity indicate another orthosis and parents are willing to continue; and
- If prescribed for use as a continued post-operative adjunct or for preventative measures.
The American Academy of Orthotists and Prosthetists' draft consensus statement on "Orthotic management of deformational plagiocephaly (AAOP, 2004) stated that "very young infants who have not developed midline head control, rolling, or sitting, may require a second orthosis to prevent regression of the head shape". The AAOP stated that a second orthosis is rarely required but may be used in cases of increased severity, extenuating circumstance (infant with multiple health issues), or a very young infant (less than 3 months). Criteria for use of a second orthosis include ill-fitting orthosis after multiple attempts to adjust, age/severity indicators with a willingness to continue by the family, post-operative adjunct/ preventative measures. The guideline also noted that termination of the orthotic treatment program is recommended, without weaning, when head shape falls within normal limits. If unresolved torticollis exists or if sleeping patterns are poor (same side as flatness), use is continued for an additional 2 to 4 weeks. Furthermore, unshunted or uncontrolled hydrocephalus as well as craniosynostosis are contraindications for cranial remolding orthosis.
Chan and colleagues (2013) noted that craniosynostosis results in characteristic skull deformations. Correction of craniosynostosis has traditionally involved an open cranial vault remodeling (CVR) procedure. A technique recently developed endoscope-assisted craniectomy (EAC) repair in conjunction with a post-operative molding helmet to guide cranial growth. Few studies compared these 2 approaches to the treatment of the various forms of craniosynostosis. These investigators presented a single institution's experience with open CVR and EAC. This study was a retrospective review of 57 patients who underwent craniosynostosis repair by either the endoscope-assisted or open techniques; and compared operating room times, blood loss, volume of transfused blood, length of hospital stay, and overall costs. The endoscopic technique was performed on younger children (4.7 months versus 10.6 months, p = 0.001), has shorter operating room times (2 hours 13 minutes versus 5 hours 42 minutes, p = 0.001), lower estimated blood loss (74.4 ml versus 280.2 ml, p = 0.001), less transfused blood (90.6 ml versus 226.9 ml), shorter hospital stays (1.2 days versus 4.9 days, p = 0.001), and decreased cost ($24,404 versus $42,744, p = 0.008) relative to the traditional open approach. The authors concluded that issues with the endoscope-assisted procedure primarily concerned the post-operative helmet regimen, specifically patient compliance (17.1 % non-compliance rate) and minor skin breakdown (5.7 %). The endoscope-assisted repair with post-operative helmet molding therapy was a cost-effective procedure with less operative risk and minimal post-operative morbidity. This was a valuable treatment option in younger patients with compliant care-givers.
Vogel and associates (2014) stated that the surgical management of infants with sagittal synostosis has traditionally relied on open CVR techniques; however, minimally invasive technologies, including EAC repair followed by helmet therapy (HT, EAC+HT), is increasingly used to treat various forms of craniosynostosis during the first year of life. These researchers determined the costs associated with EAC+HT in comparison with those for CVR. They performed a retrospective case-control analysis of 21 children who had undergone CVR and 21 who had undergone EAC+HT. Eligibility criteria included an age less than 1 year and at least 1 year of clinical follow-up data. Financial and clinical records were reviewed for data related to length of hospital stay and transfusion rates as well as costs associated with physician, hospital, and outpatient clinic visits. The average age of patients who underwent CVR was 6.8 months compared with 3.1 months for those who underwent EAC+HT. Patients who underwent EAC+HT most often required the use of 2 helmets (76.5 %), infrequently required a 3ird helmet (13.3 %), and averaged 1.8 clinic visits in the first 90 days after surgery. Endoscope-assisted craniectomy plus HT was associated with shorter hospital stays (mean of 1.10 versus 4.67 days for CVR, p < 0.0001), a decreased rate of blood transfusions (9.5 % versus 100 % for CVR, p < 0.0001), and a decreased operative time (81.1 versus 165.8 minutes for CVR, p < 0.0001). The overall cost of EAC+HT, accounting for hospital charges, professional and helmet fees, and clinic visits, was also lower than that of CVR ($37,255.99 versus $56,990.46, respectively, p < 0.0001). The authors concluded that EAC+HT were a less costly surgical option for patients than CVR. Furthermore, EAC+HT were associated with a lower utilization of peri-operative resources. The authors stated that these findings suggested that EAC+HT for infants with sagittal synostosis may be a cost-effective 1st-line surgical option.
Hinchcliff et al (2013) stated that the current treatment of craniosynostosis is open surgical excision of the prematurely fused suture and CVR. Due to the change in skull morphology and the increase in volume, some tension on the skin flaps is noted with closure. Although complete wound breakdown is rare, it can be a devastating complication. These researchers presented their experience with the use of the SPY imaging system (Lifecell Corporation, Branchburg, NJ) to visualize and record blood flow within the flaps of a 1-year old patient with anterior plagiocephaly. The authors concluded that intra-operative indocyanine green angiography has the potential to be a significant advantage in such cases, providing a safe and objective method to assess intra-operative scalp perfusion, allowing the surgeon to take additional measures to ameliorate any ischemic problems.
Xia et al (2008) reported on a systematic evidence review to compare molding helmet therapy with head repositioning therapy for infants with deformational plagiocephaly. The Cochrane Library and MEDLINE were searched using reported terms. Electronic searches were conducted of ISI Web of Science, Science Direct. Journals@Ovid and conference proceedings were screened. Studies that compared molding helmet therapy with head repositioning therapy for otherwise healthy infants with deformational plagiocephaly with or without torticollis were eligible for inclusion. Infants had to have received no prior treatment. Reasons for exclusion of identified studies included insufficient information about recruitment of samples and methods used to measure outcomes. The review assessed treatment success. Included studies compared molding with repositioning with and without physiotherapy or neck stretching. In most studies, the duration of treatment ranged from three to five months. All infants were under 12 months when treatment started; in most studies treatment started at five to eight months. Two reviewers independently selected studies. Seven cohort studies were included (n = 881). The number of children in each treatment group ranged from 10 to 176. Five prospective, one retrospective and one study with a prospective repositioning group and a retrospective molding group were included. All studies included consecutive infants. Flaws included allocation based on physician or patient preference, cross-over from repositioning to molding, inadequate details of co-interventions, lack of reporting of masked outcome assessment, molding offered to older or more severely affected infants and a high drop-out rate. Five studies with comparable data reported that success rates were higher in infants treated with molding compared to repositioning therapy. Of the other two studies, the average treatment time for reposition was much greater than the duration of molding time and the other did not use the same anatomical landmarks to assess outcomes in both groups. The only study (n=335) for which the author felt able to calculate the magnitude to treatment effect reported that treatment success was significantly more common in the molding compared to the repositioning group; RR 1.3 (95 % confidence interval (CI): 1.2 to 1.4); NNT 5 (95 % CI: 4 to 7). Reasons for exclusion of other studies included inadequate data or information about treatments, significant measurement bias and recruitment only of children who failed repositioning. The authors concluded that there was considerable evidence that molding therapy may be more effective at reducing skull asymmetry than repositioning therapy in infants with deformational plagiocephaly. However, studies were potentially biased and more research was required.
A critique of this systemic review stated that Xia et al’s conclusions that there was considerable evidence appeared inconsistent with the subsequent statement about potential biases in the included studies and a more cautious initial statement would appear to have been more appropriate (CRD, 2009).
Taylor et al (2015) reported long-term aesthetic outcomes with fronto-orbital advancement and CVR in treating unicoronal synostosis over a 35-year period. These investigators performed a retrospective review on patients with isolated unicoronal synostosis from 1977 to 2012. Demographic, pre-operative phenotypic, and long-term aesthetic outcomes data were analyzed with chi-squared and Fisher's exact test for categorical data and Wilcoxon rank-sum and Kruskal-Wallis rank for continuous data. A total of 238 patients were treated; 207 met inclusion criteria. None underwent secondary intervention for intracranial pressure. At definitive intervention, there were 96 (55 %) Whitaker class I patients, 11 (6 %) class II, 62 (35 %) class III, and 6 (3 %) class IV. Nasal root deviation and occipital bossing each conferred an increased risk of Whitaker class III/IV [odds ratios (OR), 4.4 (1.4 to 13.9), p = 0.011; OR, 2.6 (1.0 to 6.8), p = 0.049]. Patients who underwent bilateral CVR with extended unilateral bandeau were less likely Whitaker class III/IV at latest follow-up compared with those undergoing strictly unilateral procedures [OR, 0.2 (0.1 to 0.7), p = 0.011]. Over-correction resulted in decreased risk of temporal hollowing [OR, 0.3 (0.1 to 1.0), p = 0.05]. Patients with 5 years or more of follow-up were more likely to develop supraorbital retrusion [OR, 7.2 (2.2 to 23.4), p = 0.001] and temporal hollowing [OR, 3.7 (1.5 to 9.6), p = 0.006] and have Whitaker class III/IV outcomes [OR, 4.9 (1.8 to 12.8), p = 0.001]. The authors concluded that traditional fronto-orbital advancement and CVR appears to mitigate risk of intracranial pressure but may lead to aesthetic shortcomings as patients mature, namely fronto-orbital retrusion and temporal hollowing.
Van Wijk et al (2014) reported on the results of the first randomized controlled trial of helmet therapy in infants with positional skull deformation. The trial determined the effectiveness of helmet therapy for positional skull deformation compared with the natural course of the condition in infants aged 5-6 months. The investigators performed a pragmatic, single blinded, randomized controlled trial (HEADS, HElmet therapy Assessment in Deformed Skulls) nested in a prospective cohort study in 29 pediatric physiotherapy practices; helmet therapy was administered at four specialized centers. Study participants were 84 infants aged 5 to 6 months with moderate to severe skull deformation, who were born after 36 weeks of gestation and had no muscular torticollis, craniosynostosis, or dysmorphic features. Participants were randomly assigned to helmet therapy (n = 42) or to natural course of the condition (n=42) according to a randomization plan with blocks of eight. Six months of helmet therapy compared with the natural course of skull deformation. In both trial arms parents were asked to avoid any (additional) treatment for the skull deformation. The primary outcome was change in skull shape from baseline to 24 months of age assessed using plagiocephalometry (anthropometric measurement instrument). Change scores for plagiocephaly (oblique diameter difference index) and brachycephaly (cranioproportional index) were each included in an analysis of covariance, using baseline values as the covariate. Secondary outcomes were ear deviation, facial asymmetry, occipital lift, and motor development in the infant, quality of life (infant and parent measures), and parental satisfaction and anxiety. Baseline measurements were performed in infants aged between 5 and 6 months, with follow-up measurements at 8, 12, and 24 months. Primary outcome assessment at 24 months was blinded. The change score for both plagiocephaly and brachycephaly was equal between the helmet therapy and natural course groups, with a mean difference of -0.2 (95 % CI:-1.6 to 1.2, p = 0.80) and 0.2 (-1.7 to 2.2, p = 0.81), respectively. Full recovery was achieved in 10 of 39 (26 %) participants in the helmet therapy group and 9 of 40 (23 %) participants in the natural course group (odds ratio 1.2, 95 % CI:0.4 to 3.3, p = 0.74). All parents reported one or more side effects. The investigators concluded, based on the equal effectiveness of helmet therapy and skull deformation following its natural course, high prevalence of side effects, and high costs associated with helmet therapy, we discourage the use of a helmet as a standard treatment for healthy infants with moderate to severe skull deformation.
An UpToDate review on “Overview of craniosynostosis” (Buchanan and Hollier, 2015) states that “In most cases, positional plagiocephaly can be treated by change in positioning. A custom-fitted helmet designed to relieve pressure on the flattened side is often used in severe cases (which are rare). However, a single-blind trial has found no difference in outcomes, including change in skull shape (plagiocephaly or brachycephaly) and full recovery, at two years of age in 84 infants with moderate to severe positional skull deformation who were randomly assigned to helmet therapy or to no therapy (natural course of the condition). In addition, a number of adverse effects were reported with helmet use, including skin irritation and parental difficulty in cuddling the infant. The trial had several limitations, including the 21 percent participation rate and exclusion of the most severe cases of positional flattening. Until further larger randomized trials that include patients with more severe positional plagiocephaly/brachycephaly are performed, we will continue to suggest helmet therapy for patients with severe or recalcitrant positional flattening”.
Utria and colleagues (2016) noted that due to the changing properties of the infant skull, there is still no clear consensus on the ideal time to surgically intervene in cases of non-syndromic craniosynostosis (NSC). These investigators shed light on how patient age at the time of surgery may affect surgical outcomes and the subsequent need for reoperation. They performed a retrospective cohort review for patients with NSC who underwent primary cranial vault remodeling between 1990 and 2013. Patients' demographic and clinical characteristics and surgical interventions were recorded. Post-operative outcomes were assessed by assigning each procedure to a Whitaker category. Multi-variate logistic regression analysis was performed to determine the relationship between age at surgery and need for minor (Whitaker I or II) versus major (Whitaker III or IV) re-operation. Odds ratios for Whitaker category by age at surgery were assigned. A total of 413 unique patients underwent cranial vault remodeling procedures for NSC during the study period. Multi-variate logistic regression demonstrated increased odds of requiring major surgical revisions (Whitaker III or IV) in patients younger than 6 months of age (OR 2.49, 95 % CI: 1.05 to 5.93), and increased odds of requiring minimal surgical revisions (Whitaker I or II) in patients older than 6 months of age (OR 2.72, 95 % CI: 1.16 to 6.41). The authors concluded that timing, as a proxy for the changing properties of the infant skull, is an important factor to consider when planning vault reconstruction in NSC. They stated that the data presented in this study demonstrated that patients operated on before 6 months of age had increased odds of requiring major surgical revisions.
Subgaleal Drain Placement After Primary Cranioplasty in Craniosynostosis
Tong and associates (2015) stated that there is no published data addressing the use of post-operative subgaleal drains in patients undergoing primary cranioplasty for craniosynostosis. These investigators conducted a retrospective chart review in this population of patients, comparing outcomes of those who received post-operative drains with those who did not. They hypothesized that the subgaleal drains can significantly reduce post-operative facial edema and decrease the length of hospital stay. These researchers conducted a retrospective chart review of all patients undergoing primary cranioplasty for craniosynostosis with subgaleal drain placement (May 2010 to March 2012). A comparison group without drain placement was matched appropriately to establish a comparison of outcomes. The authors examined if subgaleal drainage led to improvement in post-operative facial edema, reduced length of hospital stay, post-operative changes in hematocrit (Hct), and complication rates. Of the 50 patients in this cohort, 25 patients had received subgaleal drains. The mean length of stay was 2.4 versus 3.5 days for the respective drained and un-drained cohorts (p = 0.03). There was no significant difference in the mean decline in Hct between drained and un-drained patients, with the mean Hct drop of 4.8 % versus 5.0 %, respectively (p = 0.83). Post-operative seroma formation developed in 3 un-drained patients (17 %) versus none in the drained cohort (0 %). Although subjective, drained patients were observed to achieve quicker resolution of facial swelling and earlier recovery of eye opening. The authors concluded that there is clinical benefit in subgaleal drain placement as earlier resolution of post-operative facial edema and a significantly shortened length of hospital stay was found among the drained cohort. They stated that future studies warrant prospective clinical trials to establish the safety and effectiveness of using subgaleal drains in cranial remodeling procedures of craniosynostosis.
Distraction Osteogenesis for Surgical Treatment of Craniosynostosis
Mundinger and colleagues (2016) stated that distraction osteogenesis has been proposed as an alternative to cranial remodeling surgery for craniosynostosis, but technique descriptions and outcome analyses are limited to small case series. These researchers summarized operative characteristics and outcomes of distraction osteogenesis and presented data comparing distraction osteogenesis to cranial remodeling surgery. They performed a systematic review of the literature; descriptive analysis, operative technical data, outcomes, or post-operative complications of distraction osteogenesis for craniosynostosis were included. A total of 1,325 citations were reviewed, yielding 53 articles and 880 children who underwent distraction osteogenesis for craniosynostosis. Distraction plates were used in 754 patients (86 %), whereas springs were used for the remaining 126 patients (14 %). Standard and spring distraction osteogenesis was reported to successfully treat the primary condition 98 % of the time. Suboptimal results were reported in 11 patients (1.3 %), and minor complications were reported in 19.5 % of cases (n = 172). Major complications were rare, occurring in 3.5 % of cases (n = 31), and included 2 reported deaths. Absolute operative times and blood loss were marginally greater for cranial remodeling surgery cases, but the differences were not statistically significant. The authors concluded that distraction osteogenesis is an effective cranial vault remodeling technique for treating craniosynostosis. No statistical differences were found with respect to operative time, blood loss, need for transfusion, or intensive care unit resources compared with cranial remodeling surgery. Moreover, they stated that outcome studies with longer follow-up periods specifically investigating cost, relapse, and reoperation rates are needed to effectively compare this treatment modality as an alternative to cranial remodeling surgery. (Level of Evidence = 4)
Pre-Operative Molding Helmet Therapy for the Treatment of Sagittal Craniosynostosis
Swanson and colleagues (2016) stated that there is no clear consensus for the optimal treatment of sagittal craniosynostosis; however, recent studies suggested that improved neurocognitive outcomes may be obtained when surgical intervention imparts active cranial expansion or remodeling and is performed before 6 months of age. The authors considered spring-mediated cranioplasty (SMC) to optimally address these imperatives, and this was an investigation of how helmet orthoses before or after SMC affect aesthetic outcomes. The authors retrospectively evaluated patients treated with SMC and adjunct helmeting for sagittal synostosis. Patients were stratified into 4 cohorts based on helmet usage:- pre-op,
- post-op,
- both, and
- neither.
The cephalic index was used to assess head shape changes and outcomes; 26 patients met inclusion criteria: 6 (23 %) had pre-op, 11 (42 %) had post-op, 4 (15 %) had pre-op and post-op, and 5 (19 %) had no helmeting. Average age at surgery was 3.6 months. Overall, cephalic index improved from a mean 69.8 to 77.9 during an average 7-month course of care. Mean pre-operative change in cephalic index showed greater improvement with pre-op helmet (1.3) versus not (0.0), (p = 0.029), despite similar initial cephalic index in these cohorts (70.4 and 69.6 respectively, p = 0.69). Nonetheless, all patient cohorts regardless of helmeting status achieved similar final cephalic indices (range of 76.4 to 80.4; p = 0.72). The authors concluded that pre-operative molding helmet therapy led to improved cephalic index at the time of spring-mediated cranioplasty. However, they noted that this benefit did not necessarily translate into overall improved cephalic index after surgery and in follow-up, calling into question the benefits of molding helmet therapy in this setting.
Post-Operative Intensive Care Unit Care Following Cranial Vault Remodeling for Sagittal Synostosis
Wolfswinkel and colleagues (2017) stated that of U.S. craniofacial and neurosurgeons, 94 % routinely admit patients to the intensive care unit (ICU) following cranial vault remodeling for correction of sagittal synostosis. These investigators examined the outcomes and cost of direct ward admission following primary cranial vault remodeling for sagittal synostosis. An institutional review board-approved retrospective review was undertaken of the records of all patients who underwent primary cranial vault remodeling for isolated sagittal craniosynostosis from 2009 to 2015 at a single pediatric hospital. Patient demographics, peri-operative course, and outcomes were recorded. A total of 110 patients met inclusion criteria with absence of other major medical problems. Average age at operation was 6.7 months, with a mean follow-up of 19.8 months; 98 patients (89 %) were admitted to a general ward for post-operative care, whereas the remaining 12 (11 %) were admitted to the ICU for pre-operative or peri-operative concerns. Among ward-admitted patients, there were 4 (3.6 %) minor complications; however, there were no major adverse events (AEs), with none necessitating ICU transfers from the ward and no mortalities. Average hospital stay was 3.7 days. The institution's financial difference in cost of ICU stay versus ward bed was $5,520 on average per bed per day. Omitting just 1 ICU post-operative day stay for this patient cohort would reduce projected health care costs by a total of $540,960 for the study period. The authors concluded that despite the common practice of post-operative admission to the ICU following cranial vault remodeling for sagittal craniosynostosis, they suggested that post-operative care be considered on an individual basis, with only a small percentage requiring a higher level of care.
Pre-Operative Erythropoietin Therapy
Fearon and Weinthal (2002) noted that the vast majority of infants and children undergoing craniosynostosis correction receive a blood transfusion. The risks of blood transfusion include, but are not limited to, acute hemolytic reactions (approximately 1 of 250,000), human immunodeficiency virus (HIV; approximately 1 of 200,000), hepatitis B and C (approximately 1 of 30,000 each), and transfusion-related lung injuries (approximately 1 of 5,000). In a prospective, single-blinded, randomized study, these researchers examined the safety and efficacy of pre-operative single weekly dosing with erythropoietin ([EPO]; epoetin alfa [Procrit]) in reducing the rate of transfusion in infants and small children undergoing craniosynostosis repair. A total of 29 patients (less than 8 years) undergoing craniosynostosis repair were randomized into 2 groups: one received pre-operative EPO (600 U/kg) weekly for 3 weeks, and the other served as a control. All care-givers responsible for blood transfusions were blinded, and strict criteria for transfusion were established. A pediatric hematologist monitored both groups, and all patients received supplemental iron (4 mg/kg); 14 patients were randomized to receive EPO, and 8 of these 14 patients (57 %) needed transfusion (mean age of 17 months; mean weight of 10.1 kg). Of the 6 patients not requiring transfusion, 3 were younger than 12 months old (mean of 6 months); 14 of 15 patients (93 %) in the control group (mean age of 13 months; mean weight of 9.3 kg) needed a blood transfusion during the study. The only control patient not requiring transfusion was the eldest (5 years old). The difference between the 2 groups was statistically significant (Fisher's exact test = 0.03). The control group showed no change in hemoglobin (Hb) levels from baseline to pre-operative levels, but the EPO group increased their average Hb levels from 12.1 to 13.1 g/dL. There were no adverse events (AEs) noted among children receiving EPO, nor were there any surgical complications. The authors concluded that the pre-operative administration of EPO significantly raised Hb levels and reduced the need for a blood transfusion with craniosynostosis correction. They stated that more suggestions were made that may further reduce the need for blood transfusions, and a cost-benefit analysis was discussed.
Koh and Gries (2007) stated that craniosynostosis, premature closures of the skull sutures, results in dysmorphic features if left untreated. Brain growth and cognitive development may also be impacted. Craniosynostosis repair is usually performed in young infants and has its peri-operative challenges. These investigators provided background information regarding the different forms of craniosynostosis, with an overview of associated anomalies, genetic influences, and their connection with cognitive function. The authors also discussed the anesthetic considerations for peri-operative management, including blood-loss management and strategies to reduce homologous blood transfusions. These researchers noted that available studies suggested that EPO may reduce the transfusion requirements, but could not eliminate the need for transfusion.
Krajewski and colleagues (2008) stated that craniosynostotic correction typically performed around infant physiologic nadir of Hb (approximately 3 to 6 months of age) is associated with high transfusion rates of packed red blood cells (RBCs) and other blood products. As a blood conserving strategy, these investigators studied the use of recombinant human EPO or Procrit (to optimize pre-operative hematocrit [Hct]); and Cell Saver (to recycle the slow, constant ooze of blood during the prolonged case). Patients with craniosynostosis at UCLA from 2003 to 2005 were divided into the study group (Procrit and Cell Saver) or the control group (n = 79). The study group first received recombinant human EPO at 3 weeks, 2 weeks, and 1 week pre-operatively and then used Cell Saver intra-operatively. Outcomes were based on morbidities and transfusion rate comparisons. The 2 groups were comparable with regards to age (5.66 and 5.71 months), and operative times (3.11 versus 2.59 hours). In the study group there was a marked increase in pre-operative Hct (56.2 %). The study group had significantly lower transfusions rates (5 % versus 100 % control group) and lower volumes transfused than in the control group (0.05 pediatric units versus 1.74 pediatric units). Additionally, of the 80 % of patients in the study group who received Cell Saver blood at the end of the case, approximately 31 % would have needed a transfusion if the recycled blood were unavailable. The authors concluded that these findings showed that for elective craniosynostotic correction, successful blood conserving dual therapy with Procrit and Cell Saver might be used to decrease transfusion rates and the need for any blood products.
Vega and associates (2014) stated that children with craniosynostosis may require cranial vault remodeling to prevent or relieve elevated intra-cranial pressure (ICP) and to correct the underlying craniofacial abnormalities. The procedure is typically associated with significant blood loss and high transfusion rates. The risks associated with transfusions were well documented and included transmission of infectious agents, bacterial contamination, acute hemolytic reactions, transfusion-related lung injury, and transfusion-related immune modulation. These investigators presented the Children's Hospital of Richmond (CHoR) protocol, which was developed to reduce the rate of blood transfusion in infants undergoing primary craniosynostosis repair. They performed a retrospective chart review of pediatric patients treated between January 2003 and February 2012. The CHoR protocol was instituted in November 2008, with the following 3 components: the use of pre-operative EPO and iron therapy; the use of an intra-operative blood recycling device: and acceptance of a lower level of Hb as a trigger for transfusion (less than 7 g/dL). Patients who underwent surgery prior to the protocol implementation served as controls. A total of 60 children were included in the study, 32 of whom were treated with the CHoR protocol. The control (C) and protocol (P) groups were comparable with respect to patient age (7 versus 8.4 months, p = 0.145). Recombinant EPO effectively raised the mean pre-operative Hb level in the P group (12 versus 9.7 g/dL, p < 0.001). Although adoption of more aggressive surgical vault re-modeling in 2008 resulted in a higher estimated blood loss (EBL; 212 versus 114.5 ml, p = 0.004) and length of surgery (4 versus 2.8 hours, p < 0.001), transfusion was performed in significantly fewer cases in the P group (56 % versus 96 %, p < 0.001). The mean length of stay (LOS) in the hospital was shorter for the P group (2.6 versus 3.4 days, p < 0.001). The authors concluded that a protocol that included pre-operative administration of recombinant EPO, intra-operative autologous blood recycling, and accepting a lower transfusion trigger significantly decreased transfusion utilization (p < 0.001). A decreased LOS (p < 0.001) was observed, although the authors did not examine if composite transfusion complication reductions led to better outcomes. These findings were confounded by the combined use of pre-operative administration of recombinant EPO, intra-operative autologous blood recycling, and accepting a lower transfusion trigger.
White and co-workers (2015) noted that surgery for craniosynostosis is associated with the potential for significant blood loss. Multiple technologies have been introduced to reduce the volume of blood transfused. These are pre-operative autologous donation; pre-operative EPO; intra-operative cell salvage (CS); acute normo-volemic hemodilution; anti-fibrinolytic drugs such as tranexamic acid, ε-aminocaproic acid, and aprotinin; fibrin sealants or fibrin glue; and post-operative drain re-infusion. All comparative studies with a treatment group and a control group were considered. There was a range of different study types from randomized controlled trials (RCTs) to case series with historic controls. These were intervention versus no intervention or a comparison of 2 interventions. Studies were identified by searching Cochrane CENTRAL, Medline, and Embase; manufacturer's Web sites; and bibliographies of relevant published articles. The primary outcome measures were the number of allogeneic blood donor exposures, the volume of allogeneic blood transfused, and the post-operative Hb or Hct levels. A total of 696 studies were identified. After removal of duplicates and after exclusion criteria were applied, 18 studies were included; 14 were case series with controls and 4 were RCTs. The authors concluded that the production of high-quality evidence on the interventions to minimize blood loss and transfusion in children undergoing surgery for craniosynostosis was difficult. Most of the literature was non-randomized and non-comparative. Several areas remain unaddressed; EPO and tranexamic acid were comparatively well studied; CS, acute normo-volemic hemodilution, and aprotinin were less so. There was only 1 comparative study on the use of fibrin glue and drain re-infusion, with no studies on pre-operative autologous donation and ε-aminocaproic acid. Tranexamic acid was clinically effective in reducing allogeneic blood transfusion. There was some evidence that CS and EPO may be clinically effective. None of the interventions studied was shown to be cost-effective because of lack of evidence.
Mathijssen (2015) stated that the “Guideline for care of patients with the diagnoses of craniosynostosis” was developed by a national working group with representatives of 11 matrix societies of specialties and the national patients’ society. All medical aspects of care for non-syndromic and syndromic craniosynostosis were included, as well as the social and psychologic impact for the patient and their parents. Managerial aspects were incorporated as well, such as organizing a timely referral to the craniofacial center, requirements for a dedicated craniofacial center, and centralization of this specialized care. The conclusions and recommendations within this document were founded on the available literature, with a grading of the level of evidence, thereby highlighting the areas of care that are in need of high-quality research. The development of this guideline was made possible by an educational grant of the Dutch Order of Medical Specialists. This guideline stated that “Administration of EPO preceding the intervention, as well as collecting autologous blood for autotransfusion are advised against”.
Peuntel-Espel and associates (2016) noted that in craniofacial centers where resources are limited, planning takes an even more important role, where potential pitfalls and complications must be extensively discussed by all team members previous to the operation. In addition to a central and an arterial line, blood transfusion products must be available and planned based on the patient's estimated blood volume and accurate blood loss monitoring. The room temperature should be kept warm, and if this is difficult, a warming device (e.g., bear hugger [3M, USA]) must be used. In addition, the use of a tumescent infiltration containing triamcinolone, ropivacaine, adrenaline, and hyaluronidase on the subgaleal plane, both at the incision site and on the regions where dissection is going to be performed, can be of invaluable help in providing hemostasis, analgesia and reducing swelling in these patients. In places where, devices such as cell saving, or medications like EPO or tranexamic acid are not available, the treating surgeons must be extremely alert of silent bleeders.
Aljaaly and colleagues (2017) stated that pediatric craniosynostosis surgery is associated with significant blood loss often requiring allogenic blood transfusion (ABT). These researchers examined the clinical effectiveness of pre-operative EPO administration in pediatric craniosynostosis surgery in reducing transfusion requirements. They carried out a systematic review and meta-analysis of the literature for studies published in English language between 1946 and 2015. Inclusion criteria included original studies in the pediatric population (0 to 8 years of age) involving pre-operative use of EPO in craniofacial procedures with quantitative reporting of peri-operative blood transfusion. Extracted data included demographics, EBL, Hb, Hct, number of patients transfused, and amount of ABT. A total of 4 studies met the inclusion criteria with a total of 117 patients. Patients were divided into 2 groups: EPO versus control. No statistical differences were found in the demographics between the 2 groups. Mean pre-operative Hct level was higher in the EPO group compared with control (43 % versus 35 %). The percentage of patients who required ABT and the volume of transfused blood were less in the EPO group (54 % versus 98 % and 84 versus 283 ml, respectively). Meta-analysis of 3 comparable studies showed a lower proportion of patients who needed blood transfusion in the EPO group. The authors concluded that the present meta-analysis demonstrated that pre-operative administration of EPO in pediatric craniosynostosis surgery decreased the proportion of patients requiring ABT. In addition, the volume of transfusion was reduced in patients who received EPO. Moreover, these investigators stated that future randomized studies are needed to establish the cost-effectiveness of routine pre-operative EPO administration in craniosynostosis surgery.
Stricker and co-workers (2017) noted that the Pediatric Craniofacial Collaborative Group established the Pediatric Craniofacial Surgery Perioperative Registry to elucidate practices and outcomes in children with craniosynostosis undergoing complex cranial vault reconstruction (CCVR) and inform quality improvement efforts. These researchers determined peri-operative management, outcomes, and complications in children undergoing CCVR across North America and to delineate salient features of current practices. A total of 31 institutions contributed data from June 2012 to September 2015. Data extracted included demographics, peri-operative management, LOS, laboratory results, and blood management techniques employed. Complications and outlier events were described. Outcomes analyzed included total blood donor exposures, intra-operative and peri-operative transfusion volumes, and LOS outcomes. A total of 1,223 cases were analyzed: 935 children aged less than or equal to 24 months and 288 children aged more than 24 months; 95 % of children aged less than or equal to 24 months and 79 % of children aged more than 24 months received at least 1 transfusion. There were no deaths. Notable complications included cardiac arrest, post-operative seizures, unplanned post-operative mechanical ventilation, large-volume transfusion, and unplanned second surgeries. Utilization of blood conservation techniques was highly variable. The authors presented a comprehensive description of peri-operative management, outcomes, and complications from a large group of North American children undergoing CCVR. These investigators stated that transfusion remained the rule for the vast majority of patients. The occurrence of numerous significant complications together with large variability in peri-operative management and outcomes suggested targets for improvement. In particular, these researchers stated that synthetic EPO has been studied as a means to increase the amount of blood loss that can be safely tolerated in infants undergoing craniofacial surgery. Despite potential benefits, only 3 patients in their dataset received pre-operative EPO. It appeared that cost, inconvenience, and concerns for complications have all but eliminated the use of EPO in CCVR.
An UpToDate review on “Red blood cell transfusion in infants and children: Indications” (Teruya, 2019) states that “Certain orthopedic surgeries are often associated with significant blood loss requiring RBC transfusion. This is particularly true of spinal fusion surgery for scoliosis, which can be associated with massive blood loss. The severity of blood loss correlates with the number of spinal levels fused. Several techniques have been used to reduce the transfusion rates in pediatric and adult patients undergoing spinal fusion. These include preoperative autologous blood donation, intraoperative blood salvage, and antifibrinolytics (e.g., tranexamic acid and epsilon-aminocaproic acid). Other surgeries that occasionally require multiple units of RBCs include certain plastic surgery and surgical oncology procedures. In particular, surgical correction of craniosynostosis almost always requires RBC transfusion. Measures that have reduced RBC allogeneic transfusions include the preoperative use of erythropoietin and perioperative blood salvage. However, in one report, acute normovolemic hemodilution in surgery for the repair of craniosynostosis did not change the need for transfusion and the volume of transfused RBC”.
Furthermore, an UpToDate review on “Overview of craniosynostosis” (Buchanan and Hollier, 2019) does not mention the use of EPO as a management tool.
Cranial Remodeling Band (or Helmet) for Calcified Cephalohematoma
Blanc and co-workers (2020) stated that cephalohematoma is a common pathology in newborns. Observation is the primary treatment for most patients with small uncomplicated cephalohematoma. Conversely, a large cephalohematoma can lead to calcification with unesthetic local deformation or deformational plagiocephaly. In a retrospective study, these researchers examined the iatrogenic risk associated with early puncture under local anesthesia and oral sucrose. This trial included a total of 67 consecutive newborns followed at Montpellier University Hospital, France, between 2010 and 2017. Large cephalohematoma was defined on the basis of the bump projection. Due to the uncertainty of the spontaneous resorption and the risk of calcification after 4 weeks that render the needle aspiration ineffective, puncture was performed between 2 and 4 weeks of life after coagulation evaluation and ultrasound (US) of the skull and scalp. Puncture was carried out in 43 boys (64 %) and 24 (36 %) girls between day 15 and day 30 after birth. The cephalohematoma maximal projection measured by US ranged from 9 to 13 mm (Q1, Q4) with a median value of 12 mm. No puncture-related complication was recorded during the intervention and at the 1-month follow-up visit. The authors concluded that in newborns with large and persistent unesthetic cephalohematoma, puncture under local anesthesia with oral sucrose can be safely proposed between day 15 and day 30 after birth.
Raines and associates (2021) stated that treatment and management of a cephalohematoma are primarily observational. The mass from a cephalohematoma takes weeks to resolve as the clotted blood is slowly absorbed. Over time, the bulge may feel harder as the collected blood calcifies. The blood then starts to be re-absorbed. Sometimes the center of the bulge begins to disappear before the edges do, giving a crater-like appearance. This is the expected course for the cephalohematoma during resolution. One should not attempt to aspirate or drain the cephalohematoma. Aspiration is not effective because the blood has clotted. In addition, entering the cephalohematoma with a needle increases the risk of infection and abscess formation. The best treatment is to leave the area alone and give the body time to re-absorb the collected fluid. Usually, cephalohematomas do not present any problem to a newborn. The exception is an increased risk of neonatal jaundice in the first days after birth; thus, the newborn needs to be carefully examined for a yellowish discoloration of the skin, sclera, or mucous membranes. Non-invasive measurements with a transcutaneous bilirubin meter can be used to screen the infant. A serum bilirubin level should be obtained if the newborn exhibits signs of jaundice. Rarely, large calcified cephalhematomas need surgical treatment.
An UpToDate review on “Skin lesions in the newborn and infant” (Mathes and Lalor, 2021) states that “Cephalohematoma presents as a swelling of the scalp that does not cross suture lines due to a subperiosteal hematoma. It is more common after instrumented delivery. Most cases resolve spontaneously over weeks, but there can be complications, such as calcification or ossification, infection, and sepsis”. Furthermore, an UpToDate review on “Neonatal birth injuries” (McKee-Garrett, 2021) states that “Calcification of the hematoma can occur with a subsequent bony swelling that may persist for months. Significant deformities of the skull may occur when calcification or ossification of the cephalohematoma occurs. Case reports have demonstrated successful surgical excision of these calcified or ossified hematomas”.
Currently, there are no published data on the use of cranial remodeling bands or helmets in the management of infants with cephalohematomas.
Anthropometric Measurements of Cranial Asymmetry
Glasgow et al (2007) noted that referrals for deformational plagiocephaly (DP) have increased, but estimates of its actual prevalence vary, depending on the population studied and criteria for diagnosis. Few studies employed an objective technique for diagnosis. These researchers validated the transcranial diameter difference (TDD) and, using it, determined the prevalence of DP among infants seen by primary care pediatricians. They determined the TDD of 32 infants referred to a cranio-facial clinic for DP; blinded to the TDD a cranio-facial surgeon assigned a DP severity score. These investigators compared the TDD and severity scores. The TDD of 192 infants presenting to primary care practices (PCP) were determined and their parents completed a DP risk factor questionnaire; ORs for associations of risk factors with DP were calculated. The correlation between TDD and DP severity score was 0.61 (p = 0.002). All infants whose TDD of greater than 0.6 cm had a severity score greater than 2; 18.2 % of the 192 infants had DP as defined by a TDD greater than 0.6 cm. Significant OR (95 % confidence intervals) for the presence of DP were sleeping supine, 3.5; (1.6, 7.5), and infant head position preference 2.2; (1.0, 4.9). Varying the sleep position decreased the risk of DP, OR = 0.40 (0.2, 0.9). The authors concluded that the TDD is a valid, objective measure of DP for use in research studies. DP is present in nearly 1 in 5 PCP infants. Because an infant who prefers to hold his head in one position is more likely to have DP, advising parents to vary the head position may reduce the risk of DP.
In a prospective study with blinded measurements, Lee et al (2008) examined the long-term effectiveness of helmet therapy in the correction of deformational plagiocephaly and evaluated the early occlusal abnormalities observed in these patients. A total of 28 patients with deformational plagiocephaly who were treated with molding helmet therapy with at least 5 years of follow-up were included in this study. The average length of molding helmet treatment was 6.2 months. At the time of this follow-up evaluation, the mean interval since completing the molding helmet therapy was 5.6 years. Anthropometric measurements of cranial asymmetry included cranial vault asymmetry (CVA), orbito-tragial depth asymmetry (OTDA), and cranial base asymmetry (CBA). A dental examination was also performed. At the completion of therapy, the most improvement was observed in the measurement of CBA, followed by CVA and OTDA. However, in evaluating the long-term stability of molding treatment, OTDA tended to continue improving after the initial treatment, while CBA and CVA appeared to regress, although none of the changes reached statistically significant levels. In dental measurements, all the dental midline and chin deviations were toward the unaffected side with respect to occipital deformation. The authors concluded that the findings of this study demonstrated that helmet remodeling with the dynamic orthotic cranioplasty band was effective in the correction of cranial asymmetry, with some non-statistically significant changes in long-term cranial vault symmetry. Dental observations indicated the possibility of occlusal abnormalities that may affect dental, especially orthodontic, diagnosis and treatment planning.
In a retrospective chart review, Graham et al (2020) focused on determining the most effective time to begin cranial remolding orthosis (CRO) treatment for infants with asymmetrical brachycephaly. Subjects with asymmetrical brachycephaly started CRO treatment between 3 and 18 months of age. These infants had a cranial vault asymmetry index (CVAI) greater than or equal to 3.5 and a cranial index (CI) greater than or equal to 90. Subjects were excluded if they had any co-morbidities affecting growth, dropped out of treatment, were lost to follow-up, or were non-compliant. Factors that were found to statistically influence treatment outcomes were subject initial age, initial CVAI, and initial CI. Overall, younger subjects were more likely to achieve a corrected head shape. The presence of prematurity or torticollis had statistically non-significant effects on the success of treatment. Initial CI was found to be a stronger predictor than initial CVAI as to which subjects achieved correction. The less severe the starting CI, the more likely the subject was to achieve full correction. The clinical understanding is that it requires more cranial growth to “round out” a full posterior skull flattening than an asymmetry. Based on the study results, infants with asymmetrical brachycephaly should be treated as early as possible to increase chances of achieving full correction of the deformity. These investigators also noted that CVAI was chosen for cranial measurement reporting due to the availability of the validated Children’s Healthcare of Atlanta (CHOA) scale. The CHOA scale defines plagiocephaly as mild when CVAI is 3.5 to 6.25, moderate when CVAI is 6.25 to 8.75, severe as a CVAI 8.75 to 11, and very severe as greater than 11. Wilbrand et al (2017) published a table of normative values of circumference, width, length, CVA, CI, and CVAI for infants from 0 to 24 months that can be used to help develop standards for defining plagiocephaly and brachycephaly. This in turn could aid in developing standards for defining asymmetrical brachycephaly. Both CI and CVAI are useful for what they were designed to measure: brachycephaly and plagiocephaly, respectively. However, there is no universal scale to measure asymmetrical brachycephaly that essentially functions as a combination of both of these deformities. Some clinicians use a combination of these scales, i.e., if a patient has a moderate CVAI and a mild CI they have a moderate deformity.
Pastor-Pons et al (2020) stated that anthropometric measurements with calipers are used to objectify cranial asymmetry in positional plagiocephaly but there is controversy regarding the reliability of different methodologies. These investigators analyzed the inter-rater and intra-rater reliability of direct anthropometric measurements with caliper on defined cranio-facial references in infants with positional plagiocephaly. From the data obtained with anthropometry, data were extracted for the calculation of cranial indices or ratios. The CI is calculated from the equation: cranial width/cranial length × 100 and determines the cranial morphology in terms of a more brachycephaly (CI greater than 85 %) or dolichocephalic skull (CI less than 75 %). On the other hand, the cranial asymmetry indices or ratios require the diagonal diameters to be determined. The most used in the bibliography is the CVAI. The CVAI is calculated with the formula: cranial diagonal diameters difference/short diagonal diameter x 100. CVAI classifies plagiocephaly severity pursuant the Children’s Healthcare of Atlanta scale: level 1: less than 3.5 %; level 2: 3.5 % to 6.25 %; level 3: 6.25 % to 8.75 %; level 4: 8.75 % to 11.0 %; level 5: greater than 11.0 %. Classification of plagiocephaly severity may guide clinicians in the decision-making process regarding the treatment options of cranial asymmetry: re-positioning, physical therapy, or cranial orthosis. Level 1 is considered within normal limits and no treatment is required. Level 2 requires re-positioning at least. Level 3 calls for cranial remolding orthosis depending on age and history and levels 4 and 5 need cranial remolding orthosis. Even with the use of cranial remolding orthosis, re-positioning and physical therapy are recommended. Early intervention translates into a significant improvement in positional plagiocephaly (PP) regardless of the severity of the asymmetries. Quantifying head shape is important for clinical management of PP and direct cranial anthropometric measurements provides an efficient solution for clinical settings.
Polycaprolactone Mesh in Pediatric Cranial Vault Remodeling Surgery
Matheus and Phua (2022) stated that the surgical management of craniosynostosis has greatly evolved with improvements in both technology and understanding of the disease process. Some limitations remain regarding bone regeneration within the surgical bony gaps. In general, bony gaps improve in the 12 to 24 months following surgery; however, some gaps may remain for longer and result in deformity and/or require additional bony reconstruction. These considerations make tissue-engineered bone very attractive. Novel 3-dimensional (3D) printed bioresorbable mesh implants made of polycaprolactone (PCL) can be used to fill the surgical bony defects. In a case-series study, these investigators examined how the use of a 3D printed biodegradable PCL mesh applied to bony defects in cranial vault surgery would affect bone healing. This study entailed 8 pediatric patients who had undergone surgical intervention using PCL mesh implants for reconstruction of bony defects during craniosynostosis correction surgery. Radiological evaluation of 3 patients at random time-points between 9 and 12 months post-operatively revealed persistent bony gaps in areas where PCL mesh was laid; 1 patient who underwent a subsequent cranial vault surgery at 9 months was found to have less bone regeneration in the defect area where PCL mesh was used when compared with an adjacent area where a particulate bone graft was used. The authors concluded that based on their experience, the use of PCL mesh on its own did not augment bone regeneration. It was possible that a greater amount of time or increased vascularization of the scaffold is needed, which supports the concept of regenerative matching axial vascularization or the further addition of osteogenic factors to increase the rate of bone formation.
References
The above policy is based on the following references:
- Abraham P, Brandel MG, Dalle Ore CL, et al. Predictors of postoperative complications of craniosynostosis repair in the national inpatient sample. Ann Plast Surg. 2018;80(5S Suppl 5):S261-S266.
- Aljaaly HA, Aldekhayel SA, Diaz-Abele J, et al. Effect of erythropoietin on transfusion requirements for craniosynostosis surgery in children. J Craniofac Surg. 2017;28(5):1315-1319.
- American Academy of Orthotists & Prosthetists (AAOP). Section 7: Orthotic treatment protocols for plagiocephaly. In: Orthotic Treatment of Deformational Plagiocephaly, Brachycephaly, and Scaphocephaly. Washington, D.C: AAOP; 2013.
- American Academy of Orthotists and Prosthetists Third Consensus Conference. Orthotic Treatment of Deformational Plagiocephaly, Brachycephaly and Scaphocephaly. J Prosthet Orthotics. 2004;16(4).
- Bialocerkowski AE, Vladusic SL, Howell SM. Conservative interventions for positional plagiocephaly: A systematic review. Develop Med Child Neurol. 2005;47(8):563-570.
- Blanc F, Bigorre M, Lamouroux A, Captier G. Early needle aspiration of large infant cephalohematoma: A safe procedure to avoid esthetic complications. Eur J Pediatr. 2020;179(2):265-269.
- Buchanan EP, Hollier LH, Jr. Overview of craniosynostosis. UpToDate [online serial]. Waltham, MA: UpToDate; reviewed February 2015.
- Buchanan EP, Hollier LH, Jr. Overview of craniosynostosis. UpToDate [online serial]. Waltham, MA: UpToDate; reviewed April 2019.
- Centre for Reviews and Dissemination (CRD). Nonsurgical treatment of deformational plagiocephaly: a systematic review. Database of Abstracts of Reviews of Effectiveness (DARE). York UK: University of York; June 17, 2009.
- Chan JW, Stewart CL, Stalder MW, et al. Endoscope-assisted versus open repair of craniosynostosis: A comparison of perioperative cost and risk. J Craniofac Surg. 2013;24(1):170-174.
- Children’s Healthcare of Atlanta. Plagiocephaly severity scale. Available at: https://pediatricapta.org/special-interest-groups/HB/ORTH_961942_PlagiocephalyScale_BWInfo.pdf.
- Cranial Technologies, Inc. Welcome to Cranial Technologies, Inc., Manufacturer of the DOC Band [website]. Tempe, AZ: Cranial Technologies; 2002. Available at: http://www.cranialtech.com/. Accessed May 16, 2002.
- de Ribaupierre S, Vernet O, Rilliet B, et al. Posterior positional plagiocephaly treated with cranial remodeling orthosis. Swiss Med Wkly. 2007;137(25-26):368-372.
- Fearon JA, Weinthal J. The use of recombinant erythropoietin in the reduction of blood transfusion rates in craniosynostosis repair in infants and children. Plast Reconstr Surg. 2002;109(7):2190-2196.
- Feijen MM, Claessens EA, Dovens AJ, et al. Babies with cranial deformity. Ned Tijdschr Geneeskd. 2009;153:A368.
- Fredrick DR, Mulliken JB, Robb RM. Ocular manifestations of deformational frontal plagiocephaly, J Pediatr Ophthalmol Strabismus. 1993;30(2):92-95.
- Gerety PA, Basta MN, Fischer JP, Taylor JA. Operative management of nonsyndromic sagittal synostosis: A head-to-head Meta-analysis of outcomes comparing 3 techniques. J Craniofac Surg. 2015;26(4):1251-1257.
- Gillette Children's Specialty Healthcare. Craniocap™ [website]. St. Paul, MN: Gillette; 2003, 2004. Available at: http://www.gillettechildrens.org/. Accessed May 13, 2004.
- Glasgow TS, Siddiqi F, Hoff C, Young PC. Deformational plagiocephaly: Development of an objective measure and determination of its prevalence in primary care. J Craniofac Surg. 2007;18(1):85-92.
- Govaert B, Michels A, Colla C, van der Hulst R. Molding therapy of positional plagiocephaly: Subjective outcome and quality of life. J Craniofac Surg. 2008;19(1):56-58.
- Graham T, Millay K, Wang J, et al. Significant factors in cranial remolding orthotic treatment of asymmetrical brachycephaly. J Clin Med. 2020;9(4):1027.
- Hinchcliff KM, Yao A, Taub PJ. Laser-assisted indocyanine green imaging to assess perfusion of scalp closure in an infant. J Craniofac Surg. 2013;24(6):2004-2006.
- Hutchison BL, Stewart AW, De Chalain TB, Mitchell EA. A randomized controlled trial of positioning treatments in infants with positional head shape deformities. Acta Paediatr. 2010;99(10):1556-1560.
- Institute for Clinical Systems Improvement (ICSI). Cranial orthoses for deformational plagiocephaly. ICSI Technology Assessment Reports. TA #082. Bloomington, MN: ICSI; March 2004.
- Koh JL, Gries H. Perioperative management of pediatric patients with craniosynostosis. Anesthesiol Clin. 2007;25(3):465-481.
- Krajewski K, Ashley RK, Pung N, et al. Successful blood conservation during craniosynostotic correction with dual therapy using procrit and cell saver. J Craniofac Surg. 2008;19(1):101-105.
- Larsen J. Orthotic treatment protocols for plagiocephaly. JPO. 2004;16(4S):31-34.
- Lee RP, Teichgraeber JF, Baumgartner JE, et al. Long-term treatment effectiveness of molding helmet therapy in the correction of posterior deformational plagiocephaly: A five-year follow-up. Cleft Palate Craniofac J. 2008;45(3):240-245.
- Lin RS, Stevens PM, Wininger M, Castiglione CL. Orthotic management of deformational plagiocephaly: Consensus Clinical Standards of Care. Cleft Palate Craniofac J. 2016;53(4):394-403.
- Masserano B, Woo AS, Skolnick GB, et al. The temporal region in unilateral coronal craniosynostosis: Fronto-orbital advancement versus endoscopy-assisted strip craniectomy. Cleft Palate Craniofac J. 2018;55(3):423-429.
- Mathes E, Lalor L. Skin lesions in the newborn and infant. UpToDate [online serial]. Waltham, MA: UpToDate; reviewed February 2021.
- Matheus IG, Phua Y. Three-dimensional printed polycaprolactone mesh in pediatric cranial vault remodeling surgery. J Craniofac Surg. 2022 Nov 21 [Online ahead of print].
- Mathijssen IMJ. Guideline for care of patients with the diagnoses of craniosynostosis: Working Group on Craniosynostosis. J Craniofac Surg. 2015;26(6):1735-1807.
- McKee-Garrett TM. Neonatal birth injuries. UpToDate [online serial]. Waltham, MA: UpToDate; reviewed February 2021.
- Moss SD. Nonsurgical, nonorthotic treatment of occipital plagiocephaly: What is the natural history of the misshapen neonatal head? J Neurosurg. 1997;87(5):667-670.
- Mundinger GS, Rehim SA, Johnson O 3rd, et al. Distraction osteogenesis for surgical treatment of craniosynostosis: A systematic review. Plast Reconstr Surg. 2016;138(3):657-669.
- NHS Quality Improvement Scotland (NHS QIS). Evidence note 16: The use of cranial orthosis treatment for infant deformational plagiocephaly. Glasgow, Scotland: NHS QIS; 2007.
- Orthomerica Products, Inc. The Global Orthotic Solution [website]. Newport Beach, CA: Orthomerica; 2002. Available at: http://www.orthomerica.com/. Accessed May 16, 2002.
- Orthomerica Products, Inc. The STARband™ Cranial Remolding Orthosis [website]. Newport Beach, CA: Orthomerica; 2003. Available at: http://www.orthomerica.com/products/cranial/starband.htm. Accessed May 13, 2004.
- Pastor-Pons I, Lucha-Lopez MO, Barrau-Lalmolda M, et al. Interrater and intrarater reliability of cranial anthropometric measurements in infants with positional plagiocephaly. Children (Basel). 2020;7(12):306.
- Persing J, James H, Swanson J, et al. Prevention and management of positional skull deformities in infants. The American Academy of Pediatrics. Clinical report. Guidance for the clinician in rendering pediatric care. Pediatrics. 2003;112(1):199-202.
- Peuntel-Espel J, Rios-Lara y Lopez RL, Moreno-Alvarez MC, Morel-Fuentes EJJ. Craniosynostosis: A multidisciplinary approach based on medical, social and demographic factors in a developing country. Revista Medica del Hospital General de México. 2016;79(4):230-239.
- Pollack IF, Losken HW, Fasick P. Diagnosis and management of posterior plagiocephaly. Pediatrics. 1997;99(2):180-185.
- Raines DA, Krawiec C, Jain S. Cephalohematoma. StatPearls [Internet]. Last updated: February 1, 2022. Available at: https://www.ncbi.nlm.nih.gov/books/NBK470192/. Accessed April 15, 2022.
- Ridgway EB, Berry-Candelario J, Grondin RT, et al. The management of sagittal synostosis using endoscopic suturectomy and postoperative helmet therapy. J Neurosurg Pediatr. 2011;7(6):620-626.
- Ripley CE, Pomatto J, Beals SP, et al. Treatment of positional plagiocephaly with dynamic orthoticcranioplasty. J Craniofacial Surg. 1994;5(3):150-159.
- Seal SK, Steinbok P, Courtemanche DJ. The cranial orbital buttress technique for nonsyndromic unicoronal and metopic craniosynostosis. Neurosurg Focus. 2015;38(5):E4.
- Seymour-Dempsey K, Baumgartner JE, Teichgraeber JF, et al. Molding helmet therapy in the management of sagittal synostosis. J Craniofac Surg. 2002;13(5):631-635.
- Stricker PA, Goobie SM, Cladis FP, et al. Perioperative outcomes and management in pediatric complex cranial vault reconstruction: A multicenter study from the Pediatric Craniofacial Collaborative Group. Anesthesiology. 2017;126:276-287.
- Swanson JW, Haas JA, Mitchell BT, et al. The effects of molding helmet therapy on spring-mediated cranial vault remodeling for sagittal craniosynostosis. J Craniofac Surg. 2016;27(6):1398-1403.
- Taylor JA, Paliga JT, Wes AM, et al. A critical evaluation of long-term aesthetic outcomes of fronto-orbital advancement and cranial vault remodeling in nonsyndromic unicoronal craniosynostosis. Plast Reconstr Surg. 2015;135(1):220-231.
- Teruya J. Red blood cell transfusion in infants and children: Indications. UpToDate [online serial]. Waltham, MA: UpToDate; reviewed April 2019.
- Tong JW, Emelin JK, Wong R, et al. Subgaleal drain placement improves surgical outcomes after primary cranioplasty in craniosynostosis patients. J Craniofac Surg. 2015;26(6):1963-1966.
- Utria AF, Lopez J, Cho RS, et al. Timing of cranial vault remodeling in nonsyndromic craniosynostosis: A single-institution 30-year experience. J Neurosurg Pediatr. 2016;18(5):629-634.
- van Wijk RM, van Vlimmeren LA, Groothuis-Oudshoorn CG, et al. Helmet therapy in infants with positional skull deformation: Randomised controlled trial. BMJ. 2014 May 1;348:g2741.
- Vega RA, Lyon C, Kierce JF, et al. Minimizing transfusion requirements for children undergoing craniosynostosis repair: The CHoR protocol. J Neurosurg Pediatr. 2014;14(2):190-195.
- Vogel TW, Woo AS, Kane AA, et al. A comparison of costs associated with endoscope-assisted craniectomy versus open cranial vault repair for infants with sagittal synostosis. J Neurosurg Pediatr. 2014;13(3):324-331.
- White N, Bayliss S, Moore D. Systematic review of interventions for minimizing perioperative blood transfusion for surgery for craniosynostosis. J Craniofac Surg. 2015;26(1):26-36.
- Wilbrand JF, Kaps K, Tabak D, et al. Normal head shape parameters in the first 2 years of life and effect of helmet therapy. Neuropediatrics. 2017;48(6):432-441.
- Wolfe A, Rubenstein A. Congenital, Syntoses. eMedicine Plastic Surgery Topic 190. Omaha, NE: eMedicine.com; updated May 14, 2003. Available at: http://www.emedicine.com/plastic/topic190.htm. Accessed December 14, 2004.
- Wolfswinkel EM, Howell LK, Fahradyan A, et al. Is postoperative intensive care unit care necessary following cranial vault remodeling for sagittal synostosis? Plast Reconstr Surg. 2017;140(6):1235-1239.
- Xia JJ, Kennedy KA, Teichgraeber JF, et al. Nonsurgical treatment of deformational plagiocephaly: A systematic review. Arch Pediatr Adolesc Med. 2008;162(8):719-727.

